Formulation Forum
Our Blog
See Dow Development Laboratories’ FormulationForum for articles and resources on various topical drug product development topics of interest.
Keeping Preservatives in Line: HPLC Method Development for Assay of Common Topical Formulation Preservatives
PURPOSE High Performance Liquid Chromatography (HPLC) assay is an integral part of topical formulation development. Method development for analysis of various analytes in topical formulations is a dynamic and challenging process. An optimal method for analysis of an...
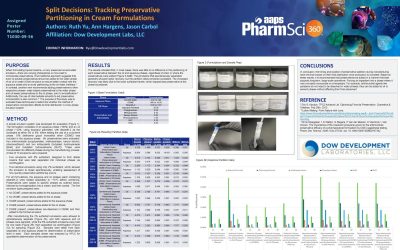
DDL Presents a Poster at AAPS 2025: Split Decisions, Tracking Preservative Partitioning in Cream Formulations
PURPOSE When formulating topical creams—or any preserved oil-and-water emulsion—there are varying philosophies on how best to incorporate preservatives. One traditional approach suggests that even oil-soluble preservatives should be added to the water phase of an...
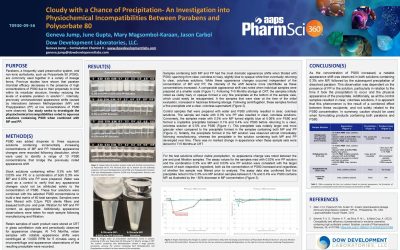
Cloudy with a Chance of Precipitation- An Investigation into Physiochemical Incompatibilities Between Parabens and Polysorbate 80
PURPOSE Parabens, a frequently used preservative system, and non-ionic surfactants, such as Polysorbate 80 (PS80), are commonly used together in a variety of dosage forms. Previous studies have shown that paraben microbial efficacy decreases in the presence of high...
Safety First: What’s Needed Before a Topical Drug Enters the Clinic
Non-clinical testing for IND submission: Before a new topical drug product can enter clinical trials, the FDA requires specific non-clinical safety studies—even if the active pharmaceutical ingredient (API) has already been approved for use via another route, such as...
Management of IP for Your Clinical Study – K.I.S.S.
Keep It Simple, Smart. Planning for investigational product (IP) supply in a clinical study is often more complex than it seems—but the guiding principle should always be to Keep It Simple, Smart (yes, we’re softening the original “stupid” just a bit!). Complexity can...
Expand/Extend Your API Life with a Topical Formulation
Let’s Topical It! Many important topical drug products started their life as oral therapies. From antifungals to NSAIDs to retinoids, metronidazole, and minoxidil—topical formulations containing these APIs have become essential treatments for fungal infections,...
Determining the Minimum Concentration of Gelling Agents to Maintain Homogeneity of Suspended API in Topical Products
Using Gravitational Force to Determine the Minimum Concentration of Various Gelling Agents Needed to Maintain Homogeneity of Micronized API in Suspension Presented by Mary Magsombol-Karaan, Jonathan Behrs, Jose Viramontes, Jake Ridgway, Geneva Jump, Nkemdilim Ekomaye,...
Improving the Robustness of Semi-Solid Formulations
Eliminating Operator Dependence from Gelling Agent Addition - Improving the Robustness and Processing Time of Semi-Solid Dosage Forms Without Impacting the Viscosity or Aesthetics Ruth Yu, Jason Carbol Dow Development Laboratories, LLC PURPOSE Aqueous gelling agents...
Determining the Most Suitable Gelling Agent for Topical Semi-Solid Formulations
View/Zoom in to view the PDF below PURPOSE Development of topical...
DDL AAPS 2025 Poster Presentation: Assessing the Impact of Cryo-Freezing in Different Topical Formulations
View/Zoom in to view the PDF below PURPOSE With recent pharmaceutical trends in the application of...
The Importance of Being Labeled Correctly
Among the myriad steps involved in pharmaceutical product development, clinical labeling and packaging stand out as critical components that need special attention. Clinical trials are typically the most expensive part of the already costly and years long...
The Use of Forced Degradation in Analytical Method Development
There are two times when forced degradation studies are needed when developing a stability-indicating method for the analysis of a drug product[1]. The first set of studies uses only the active pharmaceutical ingredient (API). Since the goal of the method is to...
Analytical Method Development for Semisolid Drug Products
The creation of an analytical method for the measurement of the API and degradation products in a semisolid formulation happens in parallel with the creation of the product itself. If the API is a repurposed drug substance, there is typically an existing method that...
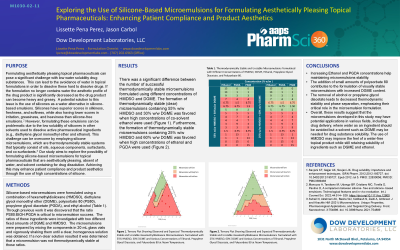
Exploring the Use of Silicone Based Microemulsions for Formulating Aesthetically Pleasing Topical Pharmaceuticals: Enhancing Patient Compliance and Product Aesthetics
PURPOSE Formulating aesthetically pleasing topical pharmaceuticals can pose a significant challenge with low water solubility drug substances. This can lead to the avoidance of water in topical formulations in order todissolve these hard to dissolve drugs. If the...
Poster Presentation at AAPS PharmSci360
Critical Material Attributes (CMA) of Excipients in Topical Formulations: The Pitfalls of Switching Between Compendial Raw Materials Without a Full Analysis of all Critical Quality Attributes (CQA) PURPOSE With the FDA introduction of Quality by Design (QbD) many...
Controlling BCC in Non-Sterile Products – A Bacteria Receiving Increased FDA Attention
Controlling the bacteria Burkholderia Cepacia Complex (BCC) in non-sterile pharmaceutical products has received increased attention in recent years. BCC has now been classified as an “objectionable organism” by the industry and will need to be controlled...
How many pea-sized amounts are you going to use?
The FDA Inactive Ingredient Database (IID) is heavily used by formulators when developing drug products. This resource has been available since 19871 and has since informed the inactive ingredient (excipient) selections and concentrations made by formulation...
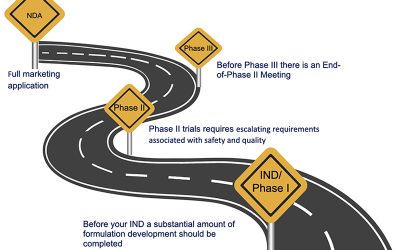
CMC Roadmap for Topical Drug Products
CMC is critical to a successful topical drug product program. Important aspects of CMC that are unique to topical products are discussed in this presentation.