Formulation Forum
Our Blog
See Dow Development Laboratories’ FormulationForum for articles and resources on various topical drug product development topics of interest.
Scale-Up of Topical Products: Math Matters
Proper scale up of a 1 kg batch to a 1,000+ kg batch takes engineering effort to execute properly. Why is this so difficult? The short answer is because of the math. The physical forces on a semi-solid product while manufacturing a 1 kg laboratory batch vs. that of...
To Suspend or Not to Suspend
Pros and Cons of topical products containing a suspended API Prescription drug products contain an active pharmaceutical ingredient (API)1. While traditional topical drug product development focuses on dissolving the API, challenges can arise from dissolved systems....
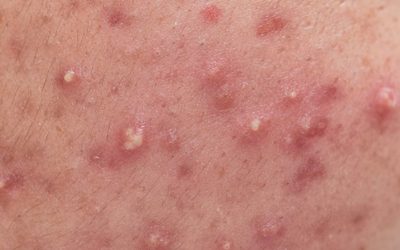
Making pimple popping a thing of the past
Acneic skin can involve inflammation, comedones, excess sebum production and bacteria. Effective topical products for the treatment of acne must be designed with patient compliance in mind. Topical Formulations Made for Patients – Acne Products When developing topical...
Ask a Formulator: What is the Purpose and Approach to Solubility Studies?
Solubility determination of the active pharmaceutical ingredient (API) in excipients suitable for topical use is a key first step in formulation development. The objective of most topical formulations is to have dissolved API so that the API can most effectively move...
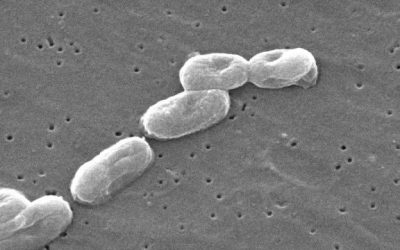
Keeping the Bugs Away
Nonsterile topical products must pass the USP/NF1 Microbial Examination of Nonsterile Products: Microbial Enumeration Tests <61> and Microbiological Examination of Nonsterile Products: Tests for Specified Microorganisms <62> and be adequately preserved. A...